Question of the Day: What is the Connection between Lead Poisoning, renal function, diabetes, and hypertension
Submitted by Norm Roulet on Thu, 04/29/2010 - 14:24.
Whether you care about childhood lead poisoning or not, I think you'll be interested by important insight on Lead, Diabetes, Hypertension, and Renal Function that everyone in the world should understand. Think of the cost of this on society - the suffering, reduced quality of life, and lost years for so many people, caused by lead harming kidney function. Here's the intro and abstract - you'll need to set up an account on WebMD to read the rest (apparently free - this is not an endorsement of WebMD... I am not a member... I will join to read the rest of this and let you know my thoughts)...
Lead, Diabetes, Hypertension, and Renal Function: The Normative Aging Study
Shirng-Wern Tsaih; Susan Korrick; Joel Schwartz; Chitra Amarasiriwardena; Antonio Aro; David Sparrow; Howard Hu
Authors and Disclosures
Posted: 09/02/2004; Environmental Health Perspectives. 2004;112(11) © 2004 National Institute of Environmental Health Sciences
Abstract and Introduction
Abstract
In this prospective study, we examined changes in renal function during 6 years of follow-up in relation to baseline lead levels, diabetes, and hypertension among 448 middle-age and elderly men, a subsample of the Normative Aging Study. Lead levels were generally low at baseline, with mean blood lead, patella lead, and tibia lead values of 6.5 µg/dL, 32.4 µg/g, and 21.5 µg/g, respectively. Six percent and 26% of subjects had diabetes and hypertension at baseline, respectively. In multivariate-adjusted regression analyses, longitudinal increases in serum creatinine (SCr) were associated with higher baseline lead levels but these associations were not statistically significant. However, we observed significant interactions of blood lead and tibia lead with diabetes in predicting annual change in SCr. For example, increasing the tibia lead level from the midpoints of the lowest to the highest quartiles (9-34 µg/g) was associated with an increase in the rate of rise in SCr that was 17.6-fold greater in diabetics than in nondiabetics (1.08 mg/dL/10 years vs. 0.062 mg/dL/10 years; p < 0.01). We also observed significant interactions of blood lead and tibia lead with diabetes in relation to baseline SCr levels (tibia lead only) and follow-up SCr levels. A significant interaction of tibia lead with hypertensive status in predicting annual change in SCr was also observed. We conclude that longitudinal decline of renal function among middle-age and elderly individuals appears to depend on both long-term lead stores and circulating lead, with an effect that is most pronounced among diabetics and hypertensives, subjects who likely represent particularly susceptible groups.
Introduction
An association between lead poisoning and renal disease in humans has been recognized for more than a century (Wedeen et al. 1975). Numerous epidemiologic studies, mortality studies, and experimental studies in animals have reported lead nephrotoxicity at high levels of exposure; however, studies on the action of lead on renal function at lower levels of chronic exposure have produced a mixed pattern of findings. Most of the studies found no significant association between low-level lead exposure and renal dysfunction. To date, only a few cross-sectional studies (Payton et al. 1994; Staessen et al. 1990, 1992) and one longitudinal study (Kim et al. 1996) have reported a significant association between elevated blood lead levels and reduced renal function measured by serum creatinine (SCr) or creatinine clearance in members of the general population. In addition, a recent randomized trial among individuals with elevated environmental lead exposure demonstrating improved creatinine clearance in those receiving chelation therapy provides evidence of lead's effect on the kidney (and its potential reversibility) at community levels of exposure (Lin et al. 2003).
Blood lead, which mostly reflects relatively recent exposure, is an inadequate measure of total body burden of lead, which may explain why most of the previous observational studies failed to find a significant association between low-level lead exposure and renal function impairment. Compared with concurrent blood lead, bone lead, which comprises > 95% of adult body lead burden and has a biologic half-life ranging from years to decades, is a better biologic marker for studying chronic toxicity of accumulated exposure and lead burden (Gonzalez-Cossio et al. 1997; Hu et al. 1996; Korrick et al. 1999). In addition, bone lead also serves as an endogenous source of lead exposure for individuals with increased bone turnover (Silbergeld 1991; Silbergeld et al. 1988). Therefore, bone lead may be a risk factor for impaired renal function either by serving as either a dosimeter of cumulative exposure of the kidney to lead or a measure of the major endogenous source of blood lead that, in turn, may affect the kidney.
Given that an increase in bone resorption is a characteristic of aging in both men and women, aging-associated release of bone lead into the circulation is a potentially important source of soft-tissue lead exposure and toxicity. Another factor associated with aging that may increase the nephrotoxicity of lead is diabetes. The more prevalent form, type 2 diabetes, affects approximately 10% or more of the general population (with substantially higher rates at ≥ 55 years of age) (Ford 2001) and is well known as an independent predictor of accelerated decline in kidney function. A third factor associated with aging that may also increase the nephrotoxicity of lead is hypertension.
In the present study, we used data from a cohort of middle-age and elderly men who had no previous known heavy lead exposure to examine the effects of low-level bone and blood lead levels on renal function. We also examined the potential modifying effect of diabetes and hypertension on these relationships.
Table 1. Table 1. Characteristics of the 707 Eligible Subjects and the 448 NAS Subjects at Baseline (1991-1995) and at Follow-Up Visit [Mean ± SD or No. (%)]
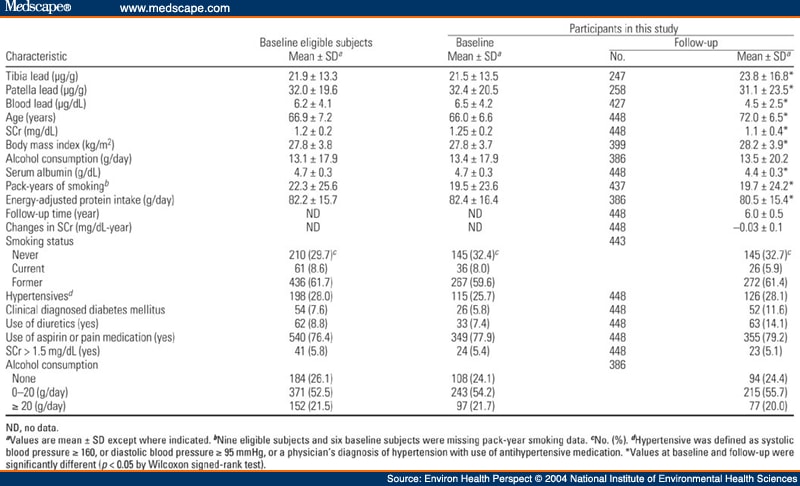
Table 2. Table 2. Multiple Regression Analysis of SCr on Blood or Bone Lead in the NAS [β(SE)]
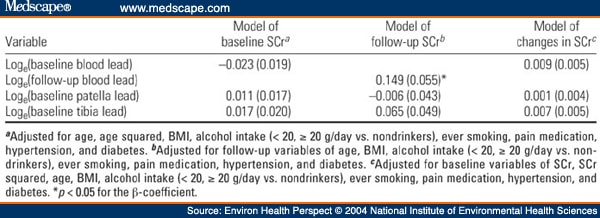
Table 3. Table 3. Multiple Regression Analysis of SCr on Blood or Bone Lead in the NAS Stratified by Baseline Diabetic Status [β(SE)]
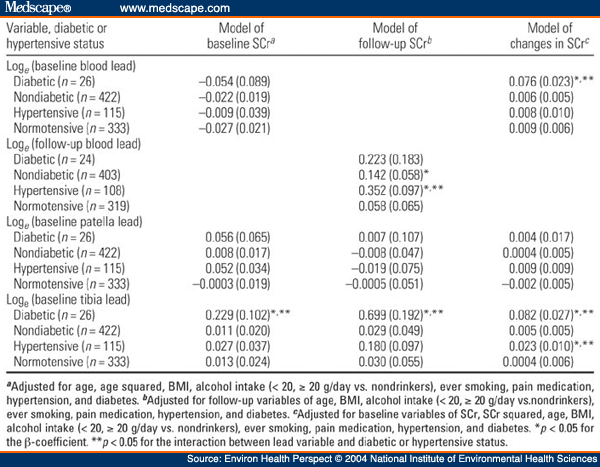
Table 3. Table 3. Multiple Regression Analysis of SCr on Blood or Bone Lead in the NAS Stratified by Baseline Diabetic Status [β(SE)]

Table 3. Table 3. Multiple Regression Analysis of SCr on Blood or Bone Lead in the NAS Stratified by Baseline Diabetic Status [β(SE)]

Table 3. Table 3. Multiple Regression Analysis of SCr on Blood or Bone Lead in the NAS Stratified by Baseline Diabetic Status [β(SE)]

Table 3. Table 3. Multiple Regression Analysis of SCr on Blood or Bone Lead in the NAS Stratified by Baseline Diabetic Status [β(SE)]

Table 3. Table 3. Multiple Regression Analysis of SCr on Blood or Bone Lead in the NAS Stratified by Baseline Diabetic Status [β(SE)]

Table 2. Table 2. Multiple Regression Analysis of SCr on Blood or Bone Lead in the NAS [β(SE)]

Table 3. Table 3. Multiple Regression Analysis of SCr on Blood or Bone Lead in the NAS Stratified by Baseline Diabetic Status [β(SE)]

Table 3. Table 3. Multiple Regression Analysis of SCr on Blood or Bone Lead in the NAS Stratified by Baseline Diabetic Status [β(SE)]

Environmental Health Perspectives. 2004;112(11) © 2004 National Institute of Environmental Health Sciences
The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Environmental Health Sciences, NIH, or the U.S. Environmental Protection Agency.
|
Update on "Lead, Diabetes, Hypertension..." from NIH
Susan Booker sent a message using the contact form at
http://realneo.us/contact.
Hi Norm,
The full text of the research paper "Lead, Diabetes, Hypertension, and
Renal Function: The Normative Aging Study" is available on the EHP website
at http://ehp.niehs.nih.gov/members/2004/7024/7024.html
The full text also can be downloaded as a pdf. No registration is required.
Hope this is helpful for your readers!
Susan M. Booker
News Editor, Environmental Health Perspectives
Disrupt IT